Abstract
A number of organisms, including dolphins, bats and electric fish, possess sophisticated active sensory systems that use self-generated signals (for example, acoustic or electrical emissions) to probe the environment1,2. Studies of active sensing in social groups have typically focused on strategies for minimizing interference from conspecific emissions2,3,4. However, it is well known from engineering that multiple spatially distributed emitters and receivers can greatly enhance environmental sensing (for example, multistatic radar and sonar)5,6,7,8. Here we provide evidence from modelling, neural recordings and behavioural experiments that the African weakly electric fish Gnathonemus petersii utilizes the electrical pulses of conspecifics to extend its electrolocation range, discriminate objects and increase information transmission. These results provide evidence for a new, collective mode of active sensing in which individual perception is enhanced by the energy emissions of nearby group members.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout

Data availability
Data reported in this study are available at Mendeley Data (https://doi.org/10.17632/jwwrfp2s78.2). Source data are provided with this paper.
Code availability
Custom code used in this work is available at https://github.com/fpedraja.
References
-
Nelson, M. E. & MacIver, M. A. Sensory acquisition in active sensing systems. J. Comp. Physiol. A 192, 573–586 (2006).
-
Jones, T. K., Allen, K. M. & Moss, C. F. Communication with self, friends and foes in active-sensing animals. J. Exp. Biol. 224, jeb242637 (2021).
-
Heiligenberg, W. Neural Nets in Electric Fish (MIT Press, 1991).
-
Rose, G. J. Insights into neural mechanisms and evolution of behaviour from electric fish. Nat. Rev. Neurosci. 5, 943–951 (2004).
-
Braca, P., Goldhahn, R., Ferri, G. & LePage, K. D. Distributed information fusion in multistatic sensor networks for underwater surveillance. IEEE Sens. J. 16, 4003–4014 (2015).
-
Chernyak, V. S. Fundamentals of Multisite Radar Systems: Multistatic Radars and Multistatic Radar Systems (CRC, 1998).
-
Boyer, F., Lebastard, V., Chevallereau, C., Mintchev, S. & Stefanini, C. Underwater navigation based on passive electric sense: new perspectives for underwater docking. Int. J. Robot. Res. 34, 1228–1250 (2015).
-
Flohr, T. G. et al. Multi-detector row CT systems and image-reconstruction techniques. Radiology 235, 756–773 (2005).
-
Heiligenberg, W. Electrolocation jamming avoidance in the mormyrid fish Brienomyrus. J. Comp. Physiol. 109, 357–372 (1976).
-
Gregg, J. D., Dudzinski, K. M. & Smith, H. V. Do dolphins eavesdrop on the echolocation signals of conspecifics? Int. J. Comp. Psychol. 20 65–88 (2007).
-
Kuc, R. Object localization from acoustic emissions produced by other sonars (L). J. Acoust. Soc. Am. 112, 1753–1755 (2002).
-
Dechmann, D. K. et al. Experimental evidence for group hunting via eavesdropping in echolocating bats. Proc. Biol. Sci. 276, 2721–2728 (2009).
-
Götz, T., Verfuß, U. K. & Schnitzler, H.-U. ‘Eavesdropping’ in wild rough-toothed dolphins (Steno bredanensis)? Biol. Lett. 2, 5–7 (2006).
-
Chiu, C., Xian, W. & Moss, C. F. Flying in silence: echolocating bats cease vocalizing to avoid sonar jamming. Proc. Natl Acad. Sci. USA 105, 13116–13121 (2008).
-
Hopkins, C. D. Neuroethology of electric communication. Annu. Rev. Neurosci. 11, 497–535 (1988).
-
Bell, C. C. Sensory coding and corollary discharge effects in mormyrid electric fish. J. Exp. Biol. 146, 229–253 (1989).
-
Kawasaki, M. in Electroreception Springer Handbook of Auditory Research (eds Bullock, T. H. et al.) Ch. 7, 154–194 (Springer, 2005).
-
Rother, D. et al. Electric images of two low resistance objects in weakly electric fish. BioSystems 71, 169–177 (2003).
-
Bell, C. C. in Electroreception (eds Bullock, T. H. & Heiligenberg, W.) 423–452 (Wiley, 1986).
-
Fortune, E. S. The decoding of electrosensory systems. Curr. Opin. Neurobiol. 16, 474–480 (2006).
-
Push, S. & Moller, P. Spatial aspects of electrolocation in the mormyrid fish, Gnathonemus petersii. J. Physiol. 75, 355–357 (1979).
-
Pedraja, F., Hofmann, V., Goulet, J. & Engelmann, J. Task-related sensorimotor adjustments increase the sensory range in electrolocation. J. Neurosci. 40, 1097–1109 (2020).
-
Babineau, D., Longtin, A. & Lewis, J. E. Modeling the electric field of weakly electric fish. J. Exp. Biol. 209, 3636–3651 (2006).
-
von der Emde, G. & Schwarz, S. Imaging of objects through active electrolocation in Gnathonemus petersii. J. Physiol. 96, 431–444 (2002).
-
Chen, L., House, J. L., Krahe, R. & Nelson, M. E. Modeling signal and background components of electrosensory scenes. J. Comp. Physiol. A 191, 331–345 (2005).
-
Baker, C. A. & Carlson, B. A. Short-term depression, temporal summation, and onset inhibition shape interval tuning in midbrain neurons. J. Neurosci. 34, 14272–14287 (2014).
-
Xu-Friedman, M. A. & Hopkins, C. D. Central mechanisms of temporal analysis in the knollenorgan pathway of mormyrid electric fish. J. Exp. Biol. 202, 1311–1318 (1999).
-
Post, N. & von der Emde, G. The ‘novelty response’ in an electric fish: response properties and habituation. Physiol. Behav. 68, 115–128 (1999).
-
Enikolopov, A. G., Abbott, L. F. & Sawtell, N. B. Internally generated Predictions enhance neural and behavioral detection of sensory stimuli in an electric fish. Neuron 99, 135–146 (2018).
-
Worm, M. et al. Evidence for mutual allocation of social attention through interactive signaling in a mormyrid weakly electric fish. Proc. Natl Acad. Sci. USA 115, 6852–6857 (2018).
-
Moller, P. Electric Fishes: History and Behavior (Chapman and Hall, 1995).
-
Russell, C. J., Myers, J. P. & Bell, C. C. The echo response in Gnathonemus petersii. J. Comp. Physiol. 92, 181–200 (1974).
-
Kramer, B. Electric organ discharge interaction during interspecific agonistic behaviour in freely swimming mormyrid fish. A method to evaluate two or more. J. Comp. Physiol. 93, 203–236 (1974).
-
Lissmann, H. W. & Machin, K. E. The mechanism of object location in Gymnarchus niloticus and similar fish. J. Exp. Biol. 35, 451–486 (1958).
-
von der Emde, G. Discrimination of objects through electrolocation in the weakly electric fish, Gnathonemus petersii. J. Comp. Physiol. A 167, 413–421 (1990).
-
Hall, J. C., Bell, C. & Zelick, R. Behavioral evidence of a latency code for stimulus intensity in mormyrid electric fish. J. Comp Physiol. A 177, 29–39 (1995).
-
Bell, C. C. Mormyromast electroreceptor organs and their afferents in mormyrid electric fish: III. Physiological differences between two morphological types of fibers. J. Neurophysiol. 63, 319–332 (1990).
-
Toerring, M. J. & Moller, P. Locomotor and electric displays associated with electrolocation during exploratory behavior in mormyrid fish. Behav. Brain Res. 12, 291–306 (1984).
-
Russell, C. J. & Bell, C. C. Neuronal responses to electrosensory input in the mormyrid valvula cerebelli. J. Neurophysiol. 41, 1495–1510 (1978).
-
Sawtell, N. B., Mohr, C. & Bell, C. C. Recurrent feedback in the mormyrid electrosensory system: cells of the preeminential and lateral toral nuclei. J. Neurophysiol. 93, 2090–2103 (2005).
-
Prechtl, J. C. et al. Sensory processing in the pallium of a mormyrid fish. J. Neurosci. 18, 7381–7393 (1998).
-
Berdahl, A., Torney, C. J., Ioannou, C. C., Faria, J. J. & Couzin, I. D. Emergent sensing of complex environments by mobile animal groups. Science 339, 574–576 (2013).
-
Moller, P. Electric signals and schooling behavior in a weakly electric fish, Marcusenius cyprinoides L. (Mormyriformes). Science 193, 697–699 (1976).
-
Worm, M., Kirschbaum, F. & von der Emde, G. Social interactions between live and artificial weakly electric fish: electrocommunication and locomotor behavior of Mormyrus rume proboscirostris towards a mobile dummy fish. PLoS ONE 12, e0184622 (2017).
-
Gebhardt, K., Alt, W. & von der Emde, G. Electric discharge patterns in group-living weakly electric fish, Mormyrus rume (Mormyridae, Teleostei). Behaviour 149, 623–644 (2012).
-
Schuster, S. Count and spark? The echo response of the weakly electric fish Gnathonemus petersii to series of pulses. J. Exp. Biol. 204, 1401–1412 (2001).
-
Bell, C. C., Han, V. & Sawtell, N. B. Cerebellum-like structures and their implications for cerebellar function. Annu. Rev. Neurosci. 31, 1–24 (2008).
-
Nieuwenhuys, R. & Nicholson, C. in Neurobiology of Cerebellar Evolution and Development (ed. Llinas, R.) 107–134 (Am. Med. Assoc, 1969).
-
Sukhum, K. V., Shen, J. & Carlson, B. A. Extreme enlargement of the cerebellum in a clade of teleost fishes that evolved a novel active sensory system. Curr. Biol. 28, 3857–3863 (2018).
-
Finger, T. E., Bell, C. C. & Russell, C. J. Electrosensory pathways to the valvula cerebelli in mormyrid fish. Exp. Brain Res. 42, 23–33 (1981).
-
Bell, C. C., Myers, J. P. & Russell, C. J. Electric organ discharge patterns during dominance related behavioral displays in Gnathonemus petersii (Mormyridae). J. Comp. Physiol. 92, 201–228 (1974).
-
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
-
Arthur, D. & Vassilvitskii, S. k-means++: the advantages of careful seeding. In Proc. 18th Annual ACM-SIAM Symposium on Discrete Algorithms, SODA'07, 1027–1035 (SIAM, 2007).
-
Braun, E., Geurten, B. & Egelhaaf, M. Identifying prototypical components in behaviour using clustering algorithms. PLoS ONE 5, e9361 (2010).
-
Migliaro, A., Caputi, A. A. & Budelli, R. Theoretical analysis of pre-receptor image conditioning in weakly electric fish. PLoS Comput. Biol. 1, 123–131 (2005).
-
Engelmann, J., Bacelo, J., van den, B. E. & Grant, K. Sensory and motor effects of etomidate anesthesia. J. Neurophysiol. 95, 1231–1243 (2006).
-
Requarth, T. & Sawtell, N. B. Plastic corollary discharge predicts sensory consequences of movements in a cerebellum-like circuit. Neuron 82, 896–907 (2014).
-
Bell, C. C., Grant, K. & Serrier, J. Corollary discharge effects and sensory processing in the mormyrid electrosensory lobe: I. Field potentials and cellular activity in associated structures. J. Neurophysiol. 68, 843–858 (1992).
-
Bell, C. C., Caputi, A. & Grant, K. Physiology and plasticity of morphologically identified cells in the mormyrid electrosensory lobe. J. Neurosci. 17, 6409–6422 (1997).
-
Mohr, C., Roberts, P. D. & Bell, C. C. Cells of the mormyromast region of the mormyrid electrosensory lobe: I. Responses to the electric organ corollary discharge and to electrosensory stimuli. J. Neurophysiol. 90, 1193–1210 (2003).
-
Kobak, D. et al. Demixed principal component analysis of neural population data. eLife 5, e10989 (2016).
Acknowledgements
This work was supported by grants from the National Institutes of Health (NS075023 and NS118448) to N.B.S. We thank L. F. Abbott, D. Turcu and A. Wallach for discussions and comments on the manuscript.
Author information
Authors and Affiliations
Contributions
N.B.S. and F.P. conceived the project, designed and carried out the experiments and wrote the manuscript. F.P. analysed the data and carried out the modelling.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Leonard Maler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Stable group behavior in Gnathonemus petersii.
a, Experimental setup for video tracking of groups consisting of one large (black) and two smaller (green and purple) Gnathonemus petersii. b, Distance between the two smaller fish and their average speed during the day (gray) and night (dashed line) (n = 5 groups). c, Stable daytime spatial configurations between two smaller fish identified using k-means cluster analysis (n = 5 groups of 3 fish). A third larger fish was also present and occupied a shelter located in the corner of the tank. For each cluster, the positions of the two fish (lines) are shown for 1000 randomly selected video frames. The heads of the fish are indicated by black dots. The number indicates the average continuous time fish spent in each configuration. d, Fish maintained closely-spaced configurations for the majority of the light-cycle in all 5 groups of fish tested (colors). Gray indicates periods when fish were >15 cm apart.
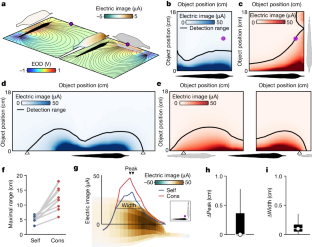
Extended Data Fig. 2 Physical characteristics of conspecific electrical images.
a, Maps of the maximal electric image generated by the fish’s own EOD (left) and the EOD of a conspecific in a right-angle configuration (right) for a 1 cm metal sphere placed at various distances and locations along the length of the fish. Black line indicates the range of object detection based on prior behavioral studies. b, Maximal electrical images as a function of distance (along the dashed line in a) for a 1 cm metal sphere with power law fits to the data. Self-images (blue), Cons-images (red). Inset shows an expanded view of the data between 10–18 cm. c, Same as in a, but with the internal conductivity of the model conspecific (dashed outline) set equal to water in order to visualize the ‘funneling’ effects of the conspecific fish’s body on electrical images. d, Difference in electric image peak location on the skin for self- and cons-images for a range of object positions (yellow) for eight different conspecific spatial configurations. Box plots show the median, the 25th to 75th percentiles and range. Related to Fig. 1g,h. e, Same as in d for electrical image width. Related to Fig. 1g,i.
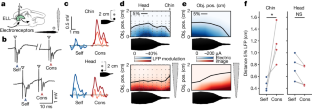
Extended Data Fig. 3 Neural correlates for electrolocation range extension by cons-EODs.
Maps of LFP amplitude modulation evoked by a 2 cm plastic rod placed at different locations near the fish (black dots). Columns are separate recording locations on the ELL map matched to the rostro-caudal locations of the object. Black lines indicate a 5% modulation of LFP amplitude. For locations near the chin appendage (left), LFP amplitude was modulated by objects at further distances for cons- (red) versus self-EODs (blue). Related to Fig. 2d,f.
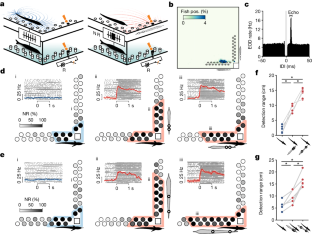
Extended Data Fig. 4 Behavioral evidence for enhanced electrolocation range based on conspecific EOD mimics.
Each set of three L-shaped plots shows NR probability for each object (grayscale) with mimic pulses off (blue; left) versus on (red; middle, right). Colored contours indicate behavioral detection range (two-tailed Binomial test: p < 0.05). Insets: top, histogram of the angular orientation of the fish for each condition across the entire ~7 day experiment. Bottom, heatmap of the distribution of fish positions in the experimental tank for each condition across the entire ~7 day experiment. In Experiment 2 the spacing between poles of the EOD mimic was increased, simulating a larger fish. Related to Fig. 3d,e.
Extended Data Fig. 5 Direction and extent of behavioral range extension matches boundary element model predictions.
a, Boundary element simulations of maximal electrical images for a 1 cm metal sphere generated by the fish’s own EOD. b, Same as in a but for cons-images generated by an EOD mimic with 4 cm spacing between electrodes used in experiment 1. Compare to behavioral results in Fig. 3d. c, Same as in a but for cons-images generated by an EOD mimic with 16 cm spacing between electrodes used in experiment 2 to simulate a larger conspecific. Compare to behavioral results in Fig. 3e.
Extended Data Fig. 6 Concurrent processing of self- and cons-EODs in ELL output cells.
a, Response of an I-type ELL output cell to modulations of the amplitude (3% steps) of a self-EOD pulse (i.e. an artificial pulse locked to the fish’s spontaneously emitted EOD motor command at a 4.5 ms delay corresponding to the naturally occurring EOD). The absolute value of the sensitivity across all 19 recorded output cells was 0.0475 ± 0.0068 spikes per 1% amplitude change (mean ± sem). b, Response of the same cell to modulations of the amplitude of cons-EOD pulses delivered across a range of delays. The absolute value of the sensitivity across all 19 recorded output cells was 0.0099 ± 0.0016 spikes per 1% amplitude change (5 ms); 0.0340 ± 0.0041 spikes per 1% amplitude change (12 ms); 0.0351 ± 0.0043 (25 ms); and 0.0387 ± 0.0049 (35 ms) (mean ± sem). c, Response of an example I-type ELL output cell to independent modulations of self- and cons-pulses (12 ms delay). Rasters and peri-stimulus time histograms illustrating responses to self- (blue) and cons-pulses (red) of different amplitudes (i and ii). Shaded rectangles indicate time windows used for quantifying responses to self- (blue) and cons-pulses (red). d, Same unit as in c. Grayscale heatmap indicates the total spike count in 100 ms window following the EOD motor command. e,f, Responses of the same unit calculated in separate time windows following self- and cons-pulses. Related to Fig. 5.
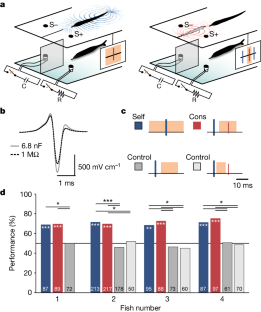
Extended Data Fig. 7 Self- and cons-electrical images provide different views of the same scene.
a, Boundary element model simulations of self- (blue) and cons- (red) electrical images of a metal cylinder (dashed) and a metal cone (solid) both 1 cm in diameter. Normalized electrical images are shown on a 3D model of the fish (bottom) and as 2D profiles (above). Black dashed lines indicate the location of the image profile on the head of the fish. Conspecific fish orientation is indicated in gray. b, Same as in a but for a different conspecific orientation and for two different orientations of a 1 cm diameter cone. Dashed and solid lines indicate the base of the cone closer and farther from the fish, respectively. In both scenarios, the spatial profiles of cons-images show greater differences (shaded area) for the two object configurations than the self-images, implying that cons-images could enhance object discrimination.
Supplementary information
Supplementary Video 1
Behaviour of a group of one dominant and two subordinate G. petersii. During the light cycle, subordinate fish often spent minutes at a time less than a body length apart in stereotyped spatial configurations, including conspicuous perpendicular, single-file and parallel line-up arrangements. See also Extended Data Fig. 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pedraja, F., Sawtell, N.B. Collective sensing in electric fish. Nature (2024). https://ift.tt/WMgb4tq
-
Received:
-
Accepted:
-
Published:
-
DOI: https://ift.tt/WMgb4tq
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
"electric" - Google News
March 06, 2024 at 11:11PM
https://ift.tt/XZThizs
Collective sensing in electric fish - Nature.com
"electric" - Google News
https://ift.tt/a8uNnIU
https://ift.tt/dEKViav
No comments:
Post a Comment