Materials scientists aren’t the first people you’d think would be pulled into the fight against COVID-19. But that’s what happened to John Rogers.
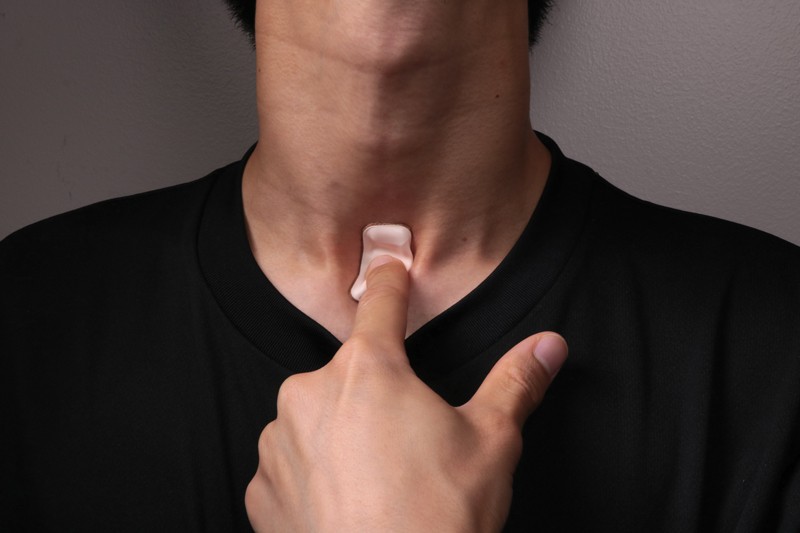
He leads a team at Northwestern University in Evanston, Illinois, that develops soft, flexible, skin-like materials with health-monitoring applications. One device, designed to sit in the hollow at the base of the throat, is a wireless, Bluetooth-connected piece of polymer and circuitry that provides real-time monitoring of talking, breathing, heart rate and other vital signs, which could be used in individuals who have had a stroke and require speech therapy1.
Physicians wanted to know whether the device could be customized to spot symptoms of the coronavirus SARS-CoV-2. The short answer was ‘yes’. Some 400 of the devices are now being used in Chicago, Illinois, to help spot early signs of COVID-19 in front-line health workers, as well as for disease monitoring in patients. His team has further tweaked the design to assess how coughing rates change in people with COVID-19.
“Several of us, designated as essential workers due to our COVID device work, have been in the lab on a daily basis throughout this period,” Rogers says. “I have not missed a day.” His team members also wear the devices in the lab, to monitor themselves for the onset of symptoms. “So far, nothing,” he says.
Rogers is one of the most prolific researchers of wearable skin-inspired electronics worldwide. This ‘e-skin’ technology has already made it into volunteers and clinics globally, helping to monitor vital signs in premature infants and hydration in athletes, for instance. Other e-skins are giving robots a lighter, human-like touch. But whether they are for people or robots, such devices represent a significant chemical and engineering challenge: electronic components are typically brittle and inflexible, and human skin is a malleable but difficult canvas.
Flexible screens, flexible circuits
E-skin devices have their roots in components found in e-book readers and curved televisions, developed by scientists working on flexible, carbon-based molecules or polymers that conduct electricity. “The organic electronics crowd was working on organic light-emitting diodes for displays and lighting, transistors for display backplanes and large-area electronics, and photovoltaic cells for solar-energy harvesting,” says George Malliaras, who studies bioelectronics at the University of Cambridge, UK. “At some point, all these applications would benefit from flexible form factors.” That flexibility, he says, “proved to be very handy when wearable electronics came to the foreground”.
One of the earliest successes in the field came in 2004. Takao Someya, an electrical engineer at the University of Tokyo, and his team reported they had developed a flexible 8 centimetre × 8 cm patch of robot skin made from layers of high-performance, pressure-sensing polyimide plastic, an organic semiconductor called pentacene and layers of gold and copper electrodes. With no silicon in sight, the square held a 32 × 32 array of tiny pressure sensors. And it allowed current to flow uninterrupted, even when it was wrapped around a 4-millimetre thick cylindrical bar, like a pliable circuit board2.
“Our team took an active matrix, developed as a drive circuit for a flexible display, to its next level,” says Someya. And it gave robots something they had never had: a sense of touch from the ability to respond to pressure.
But skin must be more than flexible, Someya realized; it must also be stretchy and conformable, and able to respond to light touch. In 2005, his team cracked that problem by spinning the relatively rigid polyimide polymer into strands and then into a net. Under tension, the strands twist, allowing the researchers to stretch the net over the surface of an egg. The stretched net was able to sense pressure changes applied to the egg from contact with a rubber block. Adding organic semiconductor diodes to the net meant it could also measure temperature3.
At Northwestern, Rogers took a different approach to the same challenge. He and his team focus on making ultrathin structures out of hard, inorganic materials, often on the nanometre scale. In 2006, the researchers worked out a way to engineer submicrometre ribbons of single-crystal silicon and bind them to a sheet of rubbery polydimethylsiloxane (PDMS) under tension. When they released the tension, the silicon deformed into undulating waves, which could flatten (but not break) as the material deformed4. “It’s sort of a hybrid organic–inorganic approach,” Rogers says.
Wear it and forget it
As Malliaras sees it, wearable devices present two types of challenge: chemistry problems in search of an engineer, and engineering problems in need of a chemist. It isn’t easy to maintain contact between an electrode and a person, because skin stretches, wrinkles and bends as people move. Gels can hold the electrode in place, but not for long, because gels are aqueous and dry out over time.
Ionic liquids represent one possible solution. Made of salts that are liquid at room temperature, ionic liquids are slow to evaporate, and good at conducting electrical current. In 2014, Malliaras and his team combined an ionic liquid called 1-ethyl-3-methylimidazolium ethyl sulfate with a polymer. This created a gel that could hold a gold electrode and a conducting polymer. The resulting device maintained its electrical performance for three days, he and his team reported5.
But such devices can also hold in sweat and block air exchange, making them irritating when worn, Someya says. They’re also fragile, meaning they cannot be used for extended periods.
To address those shortcomings, in 2017 Someya and his group hit on the idea of a porous sensor, using a mesh of flexible, gold fibres just 300–500-nm thick6. They spun a spaghetti-like mesh of polyvinyl alcohol (PVA), onto which they deposited a pattern of gold circuitry. Rinsing with water washed away the PVA to leave a flexible, gas-permeable and non-inflammatory array of wires that users can hardly tell they are wearing. Last year, the team reported using one such design to measure the beating of the human heart through the vibrations it induces in the chest — called ‘seismocardiography’ — over a period of 10 hours7.
“You can be wearing a device all day and forget that it’s there,” says Malliaras. And the benefits for health monitoring are clear. “The biggest advantage to this endeavour would be to baseline your health. Then you would be able to notice if that baseline started drifting, [and] so hopefully catch disease at the very early stages.”
Coding sensitivity
At Stanford University in California, polymer chemist Zhenan Bao is also developing electronic skins. But rather than creating sensors and then making them compatible with skin, she takes a molecular approach: designing organic polymers and electronic components with flexibility in mind from the outset.
“We design them from the molecular level and the skin-like property becomes an intrinsic property of the new material,” she says.
The applications are wide-ranging. Bao has developed a prototype device to sense hormone changes in sweat, specifically levels of cortisol — an important indicator of stress, which could be used to help understand anxiety and depression. But the technology could also be used to create organic electronics placed inside the body to help mend damaged nerves, and that morph as the body changes8.
“Over the past five or ten years, we really were able to go from initially zero materials available, to now, where we are building every component that the traditional inorganic electronics can build, but with nice skin-like materials,” Bao says.
Bao creates her materials from a range of polymers that have different conducting properties and biodegradability. In 2010, she and her team developed a skin from the elastic polymer PDMS that could detect tiny changes in pressure to mimic the sense of touch. A square of the material with tiny pyramid shapes moulded into it acts as a capacitor. When deformed, the material’s capacitance changes, and when it is pasted onto an organic transistor, changes in current can be read out — the electronic equivalent of sensing touch or pressure9. The team went on to craft its technology into a glove that could gently press on a raspberry without squashing it.
Since then, Bao has further developed the concept to make sensors that can work inside the body. In one 2019 report10, she and her team described a wireless, biodegradable sensor that could be wrapped around blood vessels and monitor blood flow continuously after surgery. To read out the signal — detected as a change in capacitance when blood pulses through the artery — the team added an external coil fitted near the skin that delivers a radio signal to a remote receiver.
Her goal, Bao says, is to make these sensors cover more of the body while maintaining cellular resolution. “We’re talking tens of micrometres resolution, but imagine over a whole body,” Bao says. “That’s extremely difficult to do with high-density electronics, and it would be too expensive for traditional silicon electronics.”
But for e-skin applications, both silicon- and organic-based approaches have their uses, Someya says. Organic electronics are suitable for large-area, low-cost, disposable applications that require moderate electronic performance, whereas silicon is suitable for high-performance, small-area applications. “Organic and inorganic are not competing with each other, but complementary,” Someya says. And in the end, he says, the most successful designs could well embody a hybrid approach, combining the best of both sets of materials.
Pain gains
In Australia, Madhu Bhaskaran at RMIT University in Melbourne favours the inorganic approach, as Rogers does.
As co-leader of a group working on functional materials and microsystems, Bhaskaran and her team use metals, such as oxides of strontium, vanadium or titanium, to develop artificial skins that can sense pain. The material could be coated onto prosthetics, for instance. Metal oxides are already widely used in electronics, she says, and have a range of applications. But they’re also brittle when heated.
In 2013, Bhaskaran’s group blended the oxide coatings with stretchy rubbers, such as silicone or PDMS, to create a stretchable electronic material11. The process isn’t straightforward. First, the researchers prepare a thin layer of patterned metal oxide on top of layers of platinum and silicon, and ‘anneal’ it at high temperatures to make the circuitry both transparent and electrically conductive. They then embed the pattern in pliable PDMS and peel it off its platinum base, leaving behind a transparent metal-oxide film. The resulting material could be stretched by up to 15% yet still maintain its electrical properties. This is thanks to tiny, tectonic-plate-like structures in the metal-oxide film, which cracks into small plates that slide over one another, allowing current to flow even when the material is deformed11.
Last year, Bhaskaran and her colleagues produced a material that can mimic the skin’s response to excessive heat, pressure and pain, as well as the brain’s reaction to it12. They combined a flexible gold–PDMS pressure sensor with a vanadium oxide temperature sensor and a component based on strontium oxide that ‘remembers’ how much electrical charge has flowed through it, called a memristor. These ‘somatosensory’ circuits fire increasingly larger signals as the intensity of the stimulus increases. “Pain is not a stimulus, pain is what our body feels when a stimulus exceeds a threshold,” Bhaskaran says. “It is the brain’s warning mechanism of danger to the body.”
Bhaskaran’s team has so far tested the material only in the lab.
Tech transfer
If that sounds like science fiction, it isn’t: some e-skin products are already in use.
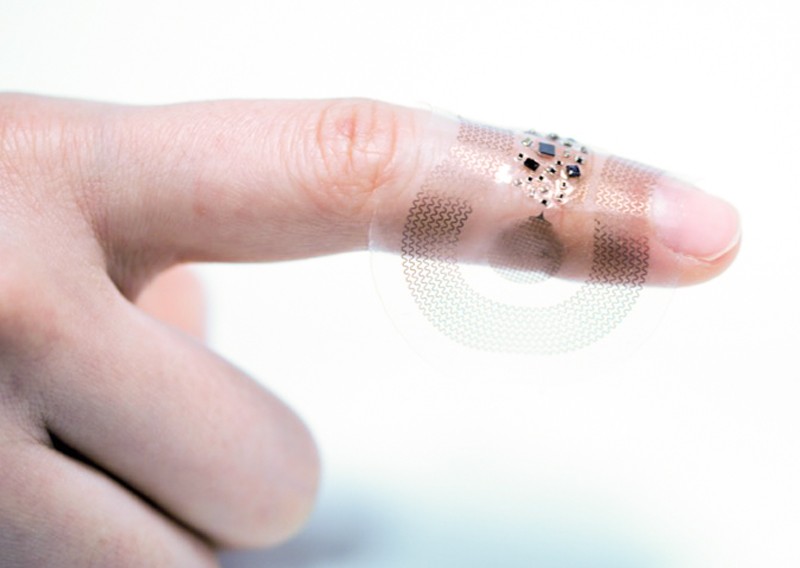
For example a sensing patch called BioStamp can be used in the home to aid clinical trials by collating huge amounts of vital signs data from participants. Developed by MC10, a company in Lexington, Massachusetts, that Rogers founded in 2008, the patch gained approval from the US Food and Drug Administration in May 2018. (MC10 was acquired by French clinical-trials company Medidata in October 2020.)
In 2019, Rogers and colleagues unveiled a sticking-plaster-sized wireless sensor that could be used to monitor premature babies in neonatal intensive care units. It has replaced the need for a tangle of monitoring wires, and made it easier for parents to hold their babies while they’re in hospital.
Rogers says around 1,000 such devices are in use in hospitals in Zambia, Ghana and Kenya, as well as at Lurie Children’s Hospital and Prentice Women’s Hospital, both in Chicago. The same hospitals are also using modified versions of the monitors to assess maternal and fetal health, Rogers says.
Someya’s group formed a spin-out company in Tokyo in 2015, called Xenoma, which uses skin-like sensors in smart clothing. This includes pyjamas that can monitor body temperature and link up to an air-conditioning unit to adjust the room temperature, or alert emergency services or family members if the wearer has a fall.
Malliaras hasn’t yet commercialized his ionic liquid technology, but is planning to test other ideas on volunteers once pandemic restrictions lift in England and allow his team to return to Addenbrooke’s Hospital in Cambridge.
Challenges
Wearable electronics are leagues ahead of the wrist-based devices that many of us use to count how many steps we take each day. For true skin-based sensitivity, prolonged and close contact with the skin is needed in a way that is impossible with these rigid, brittle, commercial devices.
That creates an intriguing suite of challenges for materials scientists. “How do you get all these materials to co-integrate and work together?” Rogers asks. Other questions include how to manage the interfaces and mechanical mismatch between hard and soft materials, he adds.
But Someya, Rogers, Bao and others’ efforts to overcome that are proving fruitful. In addition to Rogers’s work on COVID-19 and neonatal care, platforms from his lab are in use in various clinical settings. These include devices for monitoring sweat biomarkers in people with cystic fibrosis, checking skin hydration in certain skin disorders and assessing UV exposure in people with melanoma, for instance. His lab has also developed wearable sensors that track pressure and temperature between the skin and a prosthesis.
Bao suggests that Rogers’s hybrid approach explains his prolific output, because it allows him to tap into existing fabrication methods. She and her team, by contrast, have had to develop new methods. “It’s longer development. But we see that this will truly change what our electronics are going to be like in the future,” she says.
Whatever approaches researchers try, Rogers sees the recent surge of interest in wearable electronics research as a transition point that could drive further exploration. “Once you begin to establish some use cases that are really impacting and improving patients’ lives”, he says, “that creates a strong motivation for additional resources to flow into the foundational research that’s happening.”
"Electronic" - Google News
March 23, 2021 at 05:43PM
https://ift.tt/3rde8qj
Electronic skin: from flexibility to a sense of touch - Nature.com
"Electronic" - Google News
https://ift.tt/3dmroCo
https://ift.tt/3bbj3jq
No comments:
Post a Comment